
Citric acid is a tricarboxylic organic acid naturally found in citrus fruits. Industrially it is produced from the fermentation of Aspergillus niger using molasses or glucose substrates. It was first isolated in 1784 through crystallization from lemon juice.
Citric acid is available in two forms: anhydrous (water-free) and monohydrate. The anhydrous form typically crystallizes from hot water, whereas the monohydrate form results from crystallization in cold water.
It is widely used as a chelating agent, pH regulator, antioxidant synergist, and acidulant in the pharmaceutical, cosmetic, and food industry.
CAS No.:77-92-9 ( Anhydrous ), 5949-29-1(Monohydrate)
Synonyms: Kyselina citronova, 2-Hydroxytricarballylic acid, 3-Carboxy-3-hydroxypentane-1,5-dioic acid, beta-Hydroxytricarballylic acid, Citricum acidum, Citric acid monoglyceride
| Physical Properties | |
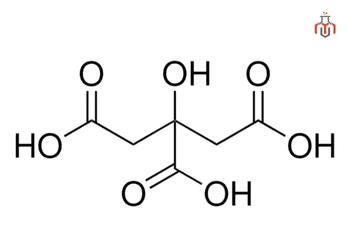
| Chemical formula | C6H8O7 (Anhydrous) |
| IUPAC name | 2-hydroxypropane-1,2,3-tricarboxylic acid |
| Molecular weight | 110.11 g/mol |
| Solubility | Very soluble in water; freely soluble in ethanol; soluble in ether |
| Odor | Odorless |
| Flash point | 100 °C |
| Density | 1.665 g/cu cm at 20 °C |
| Chemical Properties | |
| Color | White or Colorless |
| State | Crystal or powder |
| Boiling point | Decomposes |
| Melting point | 153 °C |
| LogP | -1.64 |
| Specific Gravity | 1.665 |
| pKa | 2.79 |
Citric acid is generally recognised as safe (GRAS) to use in food and skincare products, but there may be of side effects with concentrated or improper use.
Mild to Moderate effects
Rare but serious side effects
| Pictograms : |
|
| Hazard Statements : | H319 Causes serious eye irritation H335 May cause respiratory irritation |
| Precautionary statements : | P261 Avoid breathing dust P264 Wash skin thoroughly after handling P271 Use only outdoors or in a well-ventilated area P280 Wear eye protection/ face protection P304 + P340 + P312 IF INHALED: Remove person to fresh air and keep comfortable for breathing. Call a POISON CENTER/ doctor if you feel unwell P305 + P351 + P338 IF IN EYES: Rinse cautiously with water for several minutes Remove contact lenses, if present, and easy to do. Continue rinsing. |
Citric acid is a tricarboxylic organic acid naturally found in citrus fruits. Industrially it is produced from the fermentation of Aspergillus niger using molasses or glucose substrates. It was first isolated in 1784 through crystallization from lemon juice.
Citric acid is available in two forms: anhydrous (water-free) and monohydrate. The anhydrous form typically crystallizes from hot water, whereas the monohydrate form results from crystallization in cold water.
It is widely used as a chelating agent, pH regulator, antioxidant synergist, and acidulant in the pharmaceutical, cosmetic, and food industry.

CAS No.:77-92-9 ( Anhydrous ), 5949-29-1(Monohydrate)
Synonyms: Kyselina citronova, 2-Hydroxytricarballylic acid, 3-Carboxy-3-hydroxypentane-1,5-dioic acid, beta-Hydroxytricarballylic acid, Citricum acidum, Citric acid monoglyceride
| Physical Properties | |
| Chemical formula | C6H8O7 (Anhydrous) |
| IUPAC name | 2-hydroxypropane-1,2,3-tricarboxylic acid |
| Molecular weight | 110.11 g/mol |
| Solubility | Very soluble in water; freely soluble in ethanol; soluble in ether |
| Odor | Odorless |
| Flash point | 100 °C |
| Density | 1.665 g/cu cm at 20 °C |
| Chemical Properties | |
| Color | White or Colorless |
| State | Crystal or powder |
| Boiling point | Decomposes |
| Melting point | 153 °C |
| LogP | -1.64 |
| Specific Gravity | 1.665 |
| pKa | 2.79 |
Citric acid is generally recognised as safe (GRAS) to use in food and skincare products, but there may be of side effects with concentrated or improper use.
Mild to Moderate effects
Rare but serious side effects
| Pictograms : |
|
| Hazard Statements : | H319 Causes serious eye irritation H335 May cause respiratory irritation |
| Precautionary statements : | P261 Avoid breathing dust P264 Wash skin thoroughly after handling P271 Use only outdoors or in a well-ventilated area P280 Wear eye protection/ face protection P304 + P340 + P312 IF INHALED: Remove person to fresh air and keep comfortable for breathing. Call a POISON CENTER/ doctor if you feel unwell P305 + P351 + P338 IF IN EYES: Rinse cautiously with water for several minutes Remove contact lenses, if present, and easy to do. Continue rinsing. |
Citric acid plays various roles in the body, helping in the Krebs cycle, which is essential for cellular metabolism, in nutrition absorption, and it also enhances mineral absorption and acts as an antioxidant.
Macschem.us is a leading supplier of high-quality citric acid food grade. To buy citric acid anhydrous or citric acid monohydrate, send an enquiry to sales@macschem.us
Citric acid is industrially synthesised from fermentation using Aspergillus niger, a type of black mold. While the mold itself is not present in the final product, individuals with mold sensitivities may experience reactions in some cases.
Citric acid is associated with weight management; it inhibits the enzyme citrate lyase, which plays a role in converting carbohydrates into stored fat.
Small and diluted amounts of citric acid are typically safe for pets, but high doses, especially anhydrous citric acid, can cause gastrointestinal irritation in dogs.
Both forms of citric acid have specific applications and uses. Anhydrous citric acid is water-free and preferred in dry, moisture-sensitive applications. While monohydrate citric acid contains one water molecule, and preferred for water-based formulations and products that require quick solubility.