
Ascorbic Acid, commonly known as Vitamin C, is a water-soluble enediol-lactone compound. It is a weak sugar acid structurally related to glucose. Naturally occurring in citrus fruits and green vegetables, and industrially produced via the Reichstein process or through a biotechnological route using Gluconobacter oxydans.
Ascorbic Acid is widely used as a natural preservative, in skincare formulations, and industrial applications, and as a common dietary supplement available in tablets, capsules, chewable tablets, and powders.
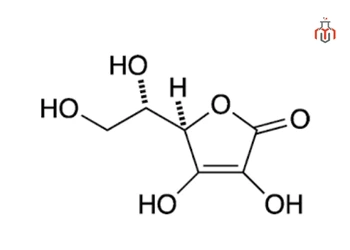
CAS No.: 50-81-7
Synonyms: L-Ascorbic acid, Vitamin C, 3-Keto-L-gulofuranolactone, Cevitamic acid
| Physical Properties | |
| Chemical formula | C6H8O6 |
| IUPAC name | (5R)-[(1S)-1,2-dihydroxyethyl]-3,4-dihydroxyfuran-2(5H)-one |
| Molecular weight | 176.12 g/mol |
| Solubility | Freely soluble in water, slightly soluble in ethanol, Insoluble in ether and chloroform |
| Odor | Odorless |
| Taste | Acidic taste |
| Density | 1.65 g/cu cm at 25 °C |
| Chemical Properties | |
| Color | White or slightly yellow |
| State | Crystal or powder |
| Storage Condition | Store in a cool, dry place, protected from light and moisture |
| Melting point | 190 °C |
| LogP | -1.85 |
| pH | 3-2 |
| pKa | 4.70 at 10 °C |
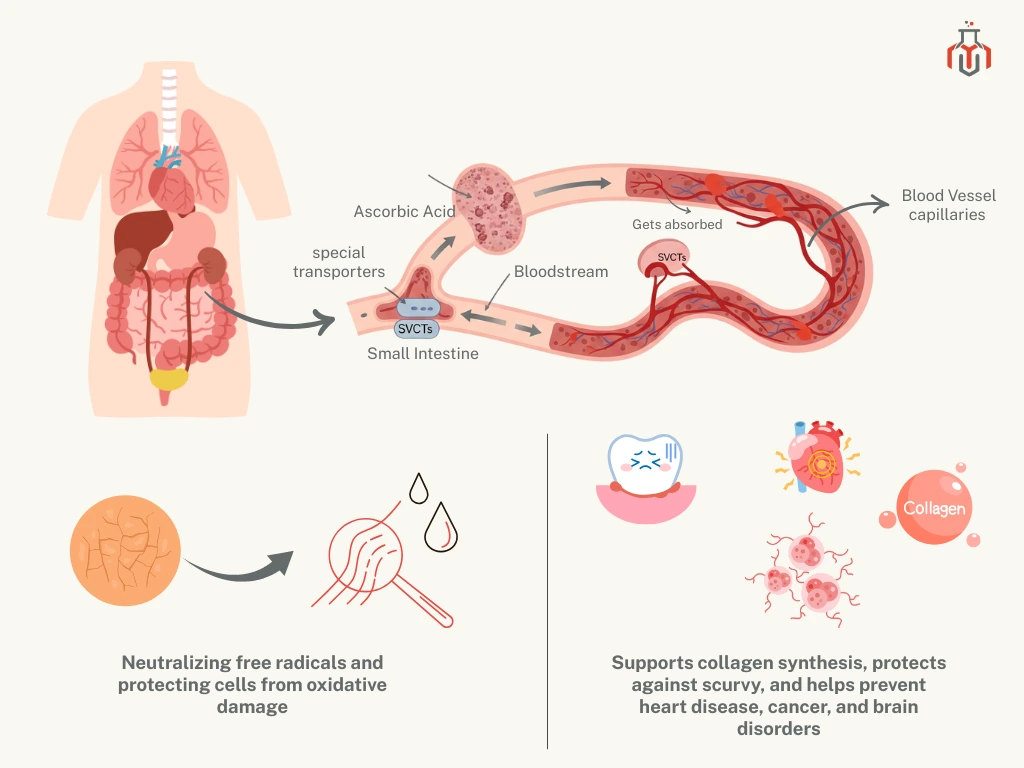
After ingestion, it gets into the gastrointestinal tract and gets absorbed into the small intestine with the help of special transporters called SVCTS (Sodium-dependent vitamin C transporters). Once absorbed, it gets distributed throughout the body via the bloodstream. It acts as a potent antioxidant, neutralizing free radicals and protecting cells from oxidative damage, which can lead to serious diseases like heart problems, cancer, and brain disorders. It also supports collagen synthesis and protects against scurvy.
Used in combination with glucose to produce Ascorbyl Glucoside, a stable derivative of L-ascorbic acid, widely used in cosmetic products.
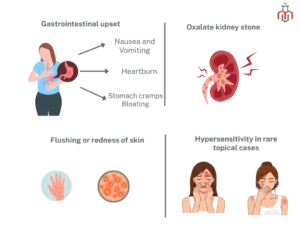
While generally safe and well-tolerated, possible side effects of ascorbic acid include:
Ascorbic Acid, commonly known as Vitamin C, is a water-soluble enediol-lactone compound. It is a weak sugar acid structurally related to glucose. Naturally occurring in citrus fruits and green vegetables, and industrially produced via the Reichstein process or through a biotechnological route using Gluconobacter oxydans.
Ascorbic Acid is widely used as a natural preservative, in skincare formulations, and industrial applications, and as a common dietary supplement available in tablets, capsules, chewable tablets, and powders.

CAS No.: 50-81-7
Synonyms: L-Ascorbic acid, Vitamin C, 3-Keto-L-gulofuranolactone, Cevitamic acid
| Physical Properties | |
| Chemical formula | C6H8O6 |
| IUPAC name | (5R)-[(1S)-1,2-dihydroxyethyl]-3,4-dihydroxyfuran-2(5H)-one |
| Molecular weight | 176.12 g/mol |
| Solubility | Freely soluble in water, slightly soluble in ethanol, Insoluble in ether and chloroform |
| Odor | Odorless |
| Taste | Acidic taste |
| Density | 1.65 g/cu cm at 25 °C |
| Chemical Properties | |
| Color | White or slightly yellow |
| State | Crystal or powder |
| Storage Condition | Store in a cool, dry place, protected from light and moisture |
| Melting point | 190 °C |
| LogP | -1.85 |
| pH | 3-2 |
| pKa | 4.70 at 10 °C |

After ingestion, it gets into the gastrointestinal tract and gets absorbed into the small intestine with the help of special transporters called SVCTS (Sodium-dependent vitamin C transporters). Once absorbed, it gets distributed throughout the body via the bloodstream. It acts as a potent antioxidant, neutralizing free radicals and protecting cells from oxidative damage, which can lead to serious diseases like heart problems, cancer, and brain disorders. It also supports collagen synthesis and protects against scurvy.
Used in combination with glucose to produce Ascorbyl Glucoside, a stable derivative of L-ascorbic acid, widely used in cosmetic products.
While generally safe and well-tolerated, possible side effects of ascorbic acid include:

Ascorbic acid has the molecular formula C₆H₈O₆, composed of carbon, hydrogen, and oxygen arranged in a furan-based lactone ring. It is synthesized using glucose as a starting material through the Reichstein process.
Ascorbic acid is beneficial for skin health and helps in reducing the signs of aging, hyperpigmentation, and sun damage. It acts as an antioxidant, protecting skin cells from the free radicals that damage them.
Ascorbic acid is generally safe, but in some individuals, it can cause acid reflux. This is because ascorbic acid can trigger the stomach acid secretion. If an individual experiences problems it is advisable to consult a healthcare professional.
Small amounts of ascorbic acid are generally safe for dogs and are common in pet supplements. However, excessive intake may lead to digestive upset. Always consult a veterinarian before use.
Ascorbic acid can help prevent botulism, a paralytic illness caused by the bacterium Clostridium botulinum, by lowering the pH of food below 4.6 and inhibiting bacterial growth.
The Safe range for ascorbic acid (Vitamin C) depends on various factors such as health condition, age, and mode of administration (oral or IV). For adults, the recommended daily intake is 75 mg for women and 90 mg for men.