
Fumaric acid is a white crystalline compound with the chemical formula HO₂CCH=CHCO₂H. It is a key ingredient in cosmetics and personal care formulations, and an approved acidity regulator in the food and beverage industry. Fumaric acid is an essential intermediate in the citric acid cycle (Krebs cycle) and helps in cellular respiration and energy production.
Its name comes from the plant Fumaria officinalis, where it was first extracted. Fumaric acid is naturally found in fruits and vegetables and industrially synthesised by the fermentation or by isomerisation reaction.
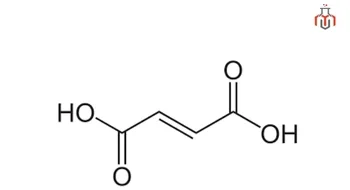
CAS No.: 110-17-8
Synonyms: Boletic acid, Allomaleic acid, Trans-butenedioic acid, Trans-1,2-Ethylenedicarboxylic acid, Lichenic acid, Tumaric acid, Fumaricum acidum
| Physical Properties | |
| Chemical formula | C4H4O4 |
| IUPAC name | (E)-but-2-enedioic acid |
| Molecular weight | 116.07 g/mol |
| Solubility | Soluble in alcohol, slightly soluble in water and in ether, and very slightly soluble in chloroform |
| Odor | Odorless |
| Density | 1.635 g/cm³ at 20°C |
| Chemical Properties | |
| Appearance | Colorless crystalline solid |
| Color | White |
| Boiling point | 522 °C |
| Melting point | 287°C |
| pH | 3.19 |
| Flash point | 230 °C |
| pKa | 3.02, 4.38(at 25℃) |
Fumaric acid is categorised as GRAS for use in foods and cosmetics. However, when consumed in higher doses (particularly in pharmaceutical fumarate formulations such as dimethyl fumarate), certain side effects may occur.
Common side effects
Prolonged use can lead to Serious side effects such as
| Pictograms : |
|
| Hazard Statements : | H319 Causes serious eye irritation |
| Precautionary statements : | P264 Wash skin thoroughly after handling P280 Wear eye protection/ face protection P305 + P351 + P338 IF IN EYES: Rinse cautiously with water for several minutes Remove contact lenses, if present and easy to do. Continue rinsing P337 + P313 If eye irritation persists: Get medical advice/ attention |
Fumaric acid is a white crystalline compound with the chemical formula HO₂CCH=CHCO₂H. It is a key ingredient in cosmetics and personal care formulations, and an approved acidity regulator in the food and beverage industry. Fumaric acid is an essential intermediate in the citric acid cycle (Krebs cycle) and helps in cellular respiration and energy production.
Its name comes from the plant Fumaria officinalis, where it was first extracted. Fumaric acid is naturally found in fruits and vegetables and industrially synthesised by the fermentation or by isomerisation reaction.

CAS No.: 110-17-8
Synonyms: Boletic acid, Allomaleic acid, Trans-butenedioic acid, Trans-1,2-Ethylenedicarboxylic acid, Lichenic acid, Tumaric acid, Fumaricum acidum
| Physical Properties | |
| Chemical formula | C4H4O4 |
| IUPAC name | (E)-but-2-enedioic acid |
| Molecular weight | 116.07 g/mol |
| Solubility | Soluble in alcohol, slightly soluble in water and in ether, and very slightly soluble in chloroform |
| Odor | Odorless |
| Density | 1.635 g/cm³ at 20°C |
| Chemical Properties | |
| Appearance | Colorless crystalline solid |
| Color | White |
| Boiling point | 522 °C |
| Melting point | 287°C |
| pH | 3.19 |
| Flash point | 230 °C |
| pKa | 3.02, 4.38(at 25℃) |

Fumaric acid is categorised as GRAS for use in foods and cosmetics. However, when consumed in higher doses (particularly in pharmaceutical fumarate formulations such as dimethyl fumarate), certain side effects may occur.
Common side effects
Prolonged use can lead to Serious side effects such as
| Pictograms : |
|
| Hazard Statements : | H319 Causes serious eye irritation |
| Precautionary statements : | P264 Wash skin thoroughly after handling P280 Wear eye protection/ face protection P305 + P351 + P338 IF IN EYES: Rinse cautiously with water for several minutes Remove contact lenses, if present and easy to do. Continue rinsing P337 + P313 If eye irritation persists: Get medical advice/ attention |
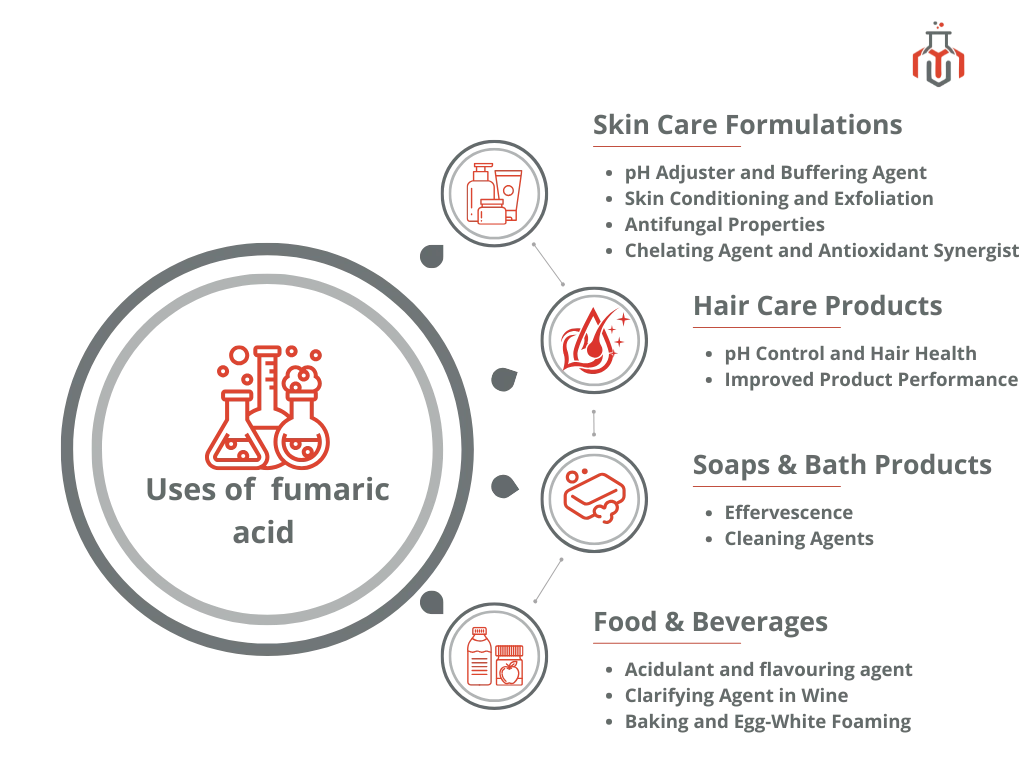
Fumaric acid used to regulate pH in cosmetics, enhance flavor in food products, and support treatments for psoriasis and multiple sclerosis. Its antioxidant properties also help protect skin and cells from oxidative stress.
Fumaric acid is made by the isomerisation of maleic acid or by the fermentation of glucose or other carbohydrates with the help of Fungi like Rhizopus nigricans and Rhizopus oryzae.
Fumaric acid and citric acid are both different compounds. Fumaric acid (C₄H₄O₄) is an unsaturated dicarboxylic acid, used in the food industry as an acidity regulator or flavor enhancer, while citric acid (C₆H₈O₇) is a tricarboxylic acid with one hydroxyl group used as a preservative and chelating agent.
Fumaric acid can be both natural and artificial. Naturally, it is found in fumaria officinalis, bolete mushrooms, Iceland moss, lichens, and the human body (intermediate in the Krebs cycle), and artificially made by the isomerization of maleic acid or maleic anhydride, and also by microbial fermentation.