
Potassium Citrate is a tribasic potassium salt of citric acid. It is a white crystalline powder, used as a food additive to regulate acidity. It is also used in cosmetics and skin care as a chelating agent and pH regulator.
Its monohydrate form is more hygroscopic than the anhydrous form preferred for beverages, jams, and gels, while the anhydrous form is most common in dry food and powdered drink mixes, and dietary supplements.
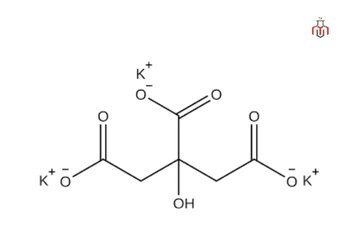
CAS No.:866-84-2
Synonyms: Kali Citricum, Hydroxycitric acid (tripotassium hydrate), Tripotassiumcitrate, Potassium 2-hydroxypropane-1,2,3-tricarboxylate, Citric Acid Tripotassium Salt, Tripotassium 2-hydroxypropane-1,2,3-tricarboxylate
| Physical Properties | |
| Chemical formula | K₃C₆H₅O₇ |
| IUPAC name | Tripotassium 2-hydroxypropane-1,2,3-tricarboxylate |
| Molecular weight | 306.39 g/mol |
| Solubility | Soluble in water and glycerol, insoluble in alcohol |
| Odor | Odorless |
| Taste | Slightly saline, cooling taste |
| Specific gravity | 1.98 g/cm³ |
| Chemical Properties | |
| Color | White |
| Appearance | Crystalline powder or granules |
| Boiling point | Decomposes before boiling |
| Melting point | ~275 °C (decomposes) |
| Hygroscopicity | Slightly hygroscopic |
| pKa | 8.5 |
| pH (1% solution) | ~7.5 – 9.0 (alkaline) |
Although generally safe, some individuals may experience side effects such as –
Rare but serious effects include gastrointestinal lesions and intestinal bleeding.
| Pictograms : | N.A |
| Hazard Statements : | N.A |
| Precautionary statements : | N.A |
Potassium Citrate is a tribasic potassium salt of citric acid. It is a white crystalline powder, used as a food additive to regulate acidity. It is also used in cosmetics and skin care as a chelating agent and pH regulator.
Its monohydrate form is more hygroscopic than the anhydrous form preferred for beverages, jams, and gels, while the anhydrous form is most common in dry food and powdered drink mixes, and dietary supplements.

CAS No.:866-84-2
Synonyms: Kali Citricum, Hydroxycitric acid (tripotassium hydrate), Tripotassiumcitrate, Potassium 2-hydroxypropane-1,2,3-tricarboxylate, Citric Acid Tripotassium Salt, Tripotassium 2-hydroxypropane-1,2,3-tricarboxylate
| Physical Properties | |
| Chemical formula | K₃C₆H₅O₇ |
| IUPAC name | Tripotassium 2-hydroxypropane-1,2,3-tricarboxylate |
| Molecular weight | 306.39 g/mol |
| Solubility | Soluble in water and glycerol, insoluble in alcohol |
| Odor | Odorless |
| Taste | Slightly saline, cooling taste |
| Specific gravity | 1.98 g/cm³ |
| Chemical Properties | |
| Color | White |
| Appearance | Crystalline powder or granules |
| Boiling point | Decomposes before boiling |
| Melting point | ~275 °C (decomposes) |
| Hygroscopicity | Slightly hygroscopic |
| pKa | 8.5 |
| pH (1% solution) | ~7.5 – 9.0 (alkaline) |
Although generally safe, some individuals may experience side effects such as –
Rare but serious effects include gastrointestinal lesions and intestinal bleeding.
| Pictograms : | N.A |
| Hazard Statements : | N.A |
| Precautionary statements : | N.A |
Potassium citrate is generally safe and considered non-hazardous. However its side effects depend upon the amount of exposure.
Potassium citrate is basic in nature; it dissociates into potassium ions (K⁺) and citrate ions (C₆H₅O₇³⁻) in aqueous solution and helps to maintain the pH level in food and beverages.
Potassium citrate is produced by the neutralization of citric acid with potassium hydroxide (KOH) or potassium carbonate (K₂CO₃). In this chemical reaction, the acidic protons of citric acid are replaced by potassium ions from KOH or K₂CO₃.
Potassium citrate is tripotassium salt of citric acid used as a food additive While potassium gluconate is potassium salt of gluconic acid. Both used as mineral supplements and food additives.