
Zinc acetate is a white crystalline salt of zinc and acetic acid, commonly found in its dihydrate form. It is primarily used in nutraceuticals and dietary supplements. As a daily oral supplement, it inhibits copper absorption and is used in the treatment of Wilson’s disease. It also exhibits antimicrobial and astringent properties, making it suitable for dermatological formulations and antiseptic preparations.
CAS No.: 557-34-6
Synonyms: Zinc diacetate Zinc(II) acetate, Dicarbomethoxyzinc, Zinc ethanoate, Acetic acid zinc salt
| Physical Properties | |
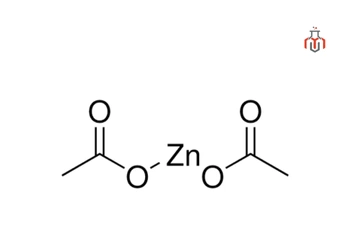
| Chemical formula | Zn(C2H3O2)2 |
| Molecular weight | 183.5 g/mol |
| Specific Gravity | 1.84 |
| Solubility | Soluble in water, dilute mineral acids and alkalies |
| Odor | Faint vinegar-like smell |
| Flash point | 12 °C |
| Density | 1.84 g/cm³ at 25 °C |
| Chemical Properties | |
| Color | White to grayish-white |
| State | Solid (Powder form) |
| Boiling point | 908°C |
| Melting point | 83-86 °C (dihydrate) |
| LogP | – 1.28 |
| Sensitivity | Moisture-sensitive (Hygroscopic) |
Common side effects include –
| Pictograms : |
|
| Hazard Statements : | H302 – Harmful if swallowed H318 – Causes serious eye damage H411 – Toxic to aquatic life with long-lasting effect |
| Precautionary statements : | P270 – Do not eat, drink, or smoke when using this product P264 – Wash face, hands, and any exposed skin thoroughly after handling P280 – Wear protective gloves/protective clothing/eye protection/face protection P305 + P351 + P338 – IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continue rinsing |
Zinc acetate is a white crystalline salt of zinc and acetic acid, commonly found in its dihydrate form. It is primarily used in nutraceuticals and dietary supplements. As a daily oral supplement, it inhibits copper absorption and is used in the treatment of Wilson’s disease. It also exhibits antimicrobial and astringent properties, making it suitable for dermatological formulations and antiseptic preparations.

CAS No.: 557-34-6
Synonyms: Zinc diacetate Zinc(II) acetate, Dicarbomethoxyzinc, Zinc ethanoate, Acetic acid zinc salt
| Physical Properties | |
| Chemical formula | Zn(C2H3O2)2 |
| Molecular weight | 183.5 g/mol |
| Specific Gravity | 1.84 |
| Solubility | Soluble in water, dilute mineral acids and alkalies |
| Odor | Faint vinegar-like smell |
| Flash point | 12 °C |
| Density | 1.84 g/cm³ at 25 °C |
| Chemical Properties | |
| Color | White to grayish-white |
| State | Solid (Powder form) |
| Boiling point | 908°C |
| Melting point | 83-86 °C (dihydrate) |
| LogP | – 1.28 |
| Sensitivity | Moisture-sensitive (Hygroscopic) |
Common side effects include –
| Pictograms : |
|
| Hazard Statements : | H302 – Harmful if swallowed H318 – Causes serious eye damage H411 – Toxic to aquatic life with long-lasting effect |
| Precautionary statements : | P270 – Do not eat, drink, or smoke when using this product P264 – Wash face, hands, and any exposed skin thoroughly after handling P280 – Wear protective gloves/protective clothing/eye protection/face protection P305 + P351 + P338 – IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continue rinsing |
Zinc acetate is a water-soluble compound and is easily absorbed by the body. However, the presence of phytates can inhibit its absorption.
Although zinc is essential for health, individuals with a zinc allergy or kidney disease should seek expert advice before using it. People with a copper deficiency should avoid zinc unless recommended by a doctor.
Zinc acetate is generally safe when used as prescribed by a healthcare professional. However, prolonged use at high doses can cause side effects such as copper deficiency.
Zinc acetate is a form of zinc that supports immune function, enhances skin health, and is used in the treatment of Wilson’s disease.
Zinc acetate is a bioavailable form of zinc beneficial for individuals with zinc deficiency and helps to maintain normal hormone balance. However, it is not a direct testosterone booster.
Zinc acetate has antimicrobial and anti-inflammatory properties and also promotes collagen production, which supports wound healing.