
Potassium acetate (CH₃COOK) is the potassium salt of acetic acid, synthesized by the neutralization reaction between potassium hydroxide (KOH) and acetic acid (CH₃COOH). It is widely used in medical formulations, dialysis solutions, pharmaceutical and biochemical research, as well as in the textile, fertilizer, and dye industries.
It is also used in the food industry as an acidity regulator and preservative due to its biocompatibility, low toxicity, and buffering properties.
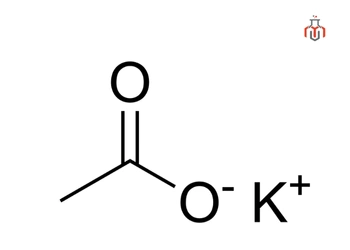
CAS No.: 127-08-2
Synonyms: Potassium ethanoate, KOAc, Kaliumacetat, Kali aceticum, potassium sal
| Physical Properties | |
| Chemical formula | CH3COOK |
| Empirical Formula | C2H3KO2 |
| Molecular weight | 98.14 g/mol |
| Solubility | Soluble in water, alcohol and liquid ammonia Insoluble in ether and acetone |
| Density | 1.6 g/cm³ |
| Flash point | N/A |
| Density | 1.6 g/cm³ |
| Chemical Properties | |
| Color | White |
| State | Crystal or powder |
| Specific Gravity | 1.57g/ml |
| Melting point | 292 °C |
| pH | 7,5-9,0 (5 % aqueous solution) |
| Vapour density (air=1) | 3.4 |
Possible side effects of potassium acetate that may occur in some individual include –
Some serious side effects of potassium acetate –
| Pictograms : |
|
| Hazard Statements : | H227 – Combustible liquid H314 – Causes severe skin burns and eye damage |
| Precautionary statements : | P210 – Keep away from heat, hot surfaces, sparks, open flames and other ignition sources. No smoking. P260 – Do not breathe dust/fume/gas/mist/vapors/spray. P264 – Wash hands, forearms and face thoroughly after handling. P280 – Wear protective gloves/protective clothing/eye protection/face protection. P301+P330+P331 – If swallowed: rinse mouth. Do NOT induce vomiting P303+P361+P353 – If on skin (or hair): Take off immediately all contaminated clothing. Rinse skin with water/shower P304+P340 – If inhaled: Remove person to fresh air and keep comfortable for breathing P305+P351+P338 – If in eyes: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continue rinsing P310 – Immediately call a poison center or doctor P321 – Specific treatment (see supplemental first aid instruction on this label) P363 – Wash contaminated clothing before reuse. P370+P378 – In case of fire: Use media other than water to extinguish. P403+P235 – Store in a well-ventilated place. Keep cool. P405 – Store locked up. P501 – Dispose of contents/container to hazardous or special waste collection point, in accordance with local, regional, national and/or international regulation
|
Potassium acetate (CH₃COOK) is the potassium salt of acetic acid, synthesized by the neutralization reaction between potassium hydroxide (KOH) and acetic acid (CH₃COOH). It is widely used in medical formulations, dialysis solutions, pharmaceutical and biochemical research, as well as in the textile, fertilizer, and dye industries.
It is also used in the food industry as an acidity regulator and preservative due to its biocompatibility, low toxicity, and buffering properties.

CAS No.: 127-08-2
Synonyms: Potassium ethanoate, KOAc, Kaliumacetat, Kali aceticum, potassium sal
| Physical Properties | |
| Chemical formula | CH3COOK |
| Empirical Formula | C2H3KO2 |
| Molecular weight | 98.14 g/mol |
| Solubility | Soluble in water, alcohol and liquid ammonia Insoluble in ether and acetone |
| Density | 1.6 g/cm³ |
| Flash point | N/A |
| Density | 1.6 g/cm³ |
| Chemical Properties | |
| Color | White |
| State | Crystal or powder |
| Specific Gravity | 1.57g/ml |
| Melting point | 292 °C |
| pH | 7,5-9,0 (5 % aqueous solution) |
| Vapour density (air=1) | 3.4 |
Possible side effects of potassium acetate that may occur in some individual include –
Some serious side effects of potassium acetate –
| Pictograms : |
|
| Hazard Statements : | H227 – Combustible liquid H314 – Causes severe skin burns and eye damage |
| Precautionary statements : | P210 – Keep away from heat, hot surfaces, sparks, open flames and other ignition sources. No smoking. P260 – Do not breathe dust/fume/gas/mist/vapors/spray. P264 – Wash hands, forearms and face thoroughly after handling. P280 – Wear protective gloves/protective clothing/eye protection/face protection. P301+P330+P331 – If swallowed: rinse mouth. Do NOT induce vomiting P303+P361+P353 – If on skin (or hair): Take off immediately all contaminated clothing. Rinse skin with water/shower P304+P340 – If inhaled: Remove person to fresh air and keep comfortable for breathing P305+P351+P338 – If in eyes: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continue rinsing P310 – Immediately call a poison center or doctor P321 – Specific treatment (see supplemental first aid instruction on this label) P363 – Wash contaminated clothing before reuse. P370+P378 – In case of fire: Use media other than water to extinguish. P403+P235 – Store in a well-ventilated place. Keep cool. P405 – Store locked up. P501 – Dispose of contents/container to hazardous or special waste collection point, in accordance with local, regional, national and/or international regulation
|
Potassium acetate is used for the treatment of low levels of potassium (hypokalemia) and metabolic acidosis . It also helps in electrolyte replacement in the diabetic ketoacidosis (DKA) and used as an additive in parenteral nutrition.
Potassium acetate is generally considered safe, however it may cause gastrointestinal upset, skin and eye irritation upon exposure. It is advisable to always follow the safety Data sheet guidelines while handling the substance.
Potassium acetate is commonly used as part of a renaturing or neutralization buffer in molecular biology, especially during DNA or plasmid extraction procedures.
Potassium acetate is not harmful when used as directed in pharmaceuticals and food-grade applications. However, excessive intake or exposure to concentrated forms may lead to irritation or potassium-related imbalances.
Potassium acetate is not found as a naturally occurring substance in whole foods, but it is added to processed meats, sauces, dairy products, and packaged foods to maintain freshness and stabilize pH.
Potassium acetate is highly soluble in water, forming a clear, slightly basic solution. This property makes it ideal for use in aqueous pharmaceutical preparations and laboratory buffers.