
Ferrous sulfate (iron (II) sulfate) is an inorganic salt used as a dietary supplement to prevent or treat iron deficiency anemia. It is typically found in its hydrated form, most notably as ferrous sulfate heptahydrate. It is included in the WHO list of Essential Medicines and is one of the most commonly prescribed iron formulations in the United States.
Ferrous sulfate works by increasing the Fe level in the body, supporting the synthesis of hemoglobin and myoglobin, which are critical for oxygen transport and its storage in blood and muscle tissues.
In the duodenum, Fe²⁺ ions are absorbed by enterocytes through Divalent Metal Transporter 1 (DMT1). Inside the cell, iron is either stored as ferritin or exported into the bloodstream by ferroportin. In circulation, it binds to transferrin for delivery to tissues and the bone marrow.
CAS No: 7720-78-7
Synonyms: Iron sulfate, Green vitriol, Ferrous sulfate heptahydrate, Ferrosulfate, Iron vitriol, Iron(II) sulfate heptahydrate, Ferrous sulfate anhydrous
| Physical Properties | |
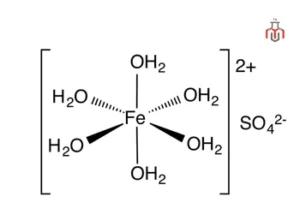
| Chemical formula | FeSO4.7H2O |
| IUPAC Name | Iron(2+) sulfate heptahydrate |
| Molecular weight | 278.01 g/mol |
| State | Crystalline Solid |
| Melting point | 64 °C |
| Density | 1.898 g/mL at 25 °C (lit.) |
| Chemical Properties | |
| Color | Blue-green |
| Solubility | Soluble in water |
| Odor | Odorless |
| pH | 3-4 |
| Pictograms : |  |
| Hazard Statements : | H302 Harmful if swallowed H315 Causes skin irritation H319 Causes serious eye irritation |
| Precautionary statements : | P305 + P351 + P338 IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present, and easy to do. Continue rinsing. |
Ferrous sulfate (iron (II) sulfate) is an inorganic salt used as a dietary supplement to prevent or treat iron deficiency anemia. It is typically found in its hydrated form, most notably as ferrous sulfate heptahydrate. It is included in the WHO list of Essential Medicines and is one of the most commonly prescribed iron formulations in the United States.

Ferrous sulfate works by increasing the Fe level in the body, supporting the synthesis of hemoglobin and myoglobin, which are critical for oxygen transport and its storage in blood and muscle tissues.
In the duodenum, Fe²⁺ ions are absorbed by enterocytes through Divalent Metal Transporter 1 (DMT1). Inside the cell, iron is either stored as ferritin or exported into the bloodstream by ferroportin. In circulation, it binds to transferrin for delivery to tissues and the bone marrow.
CAS No: 7720-78-7
Synonyms: Iron sulfate, Green vitriol, Ferrous sulfate heptahydrate, Ferrosulfate, Iron vitriol, Iron(II) sulfate heptahydrate, Ferrous sulfate anhydrous
| Physical Properties | |
| Chemical formula | FeSO4.7H2O |
| IUPAC Name | Iron(2+) sulfate heptahydrate |
| Molecular weight | 278.01 g/mol |
| State | Crystalline Solid |
| Melting point | 64 °C |
| Density | 1.898 g/mL at 25 °C (lit.) |
| Chemical Properties | |
| Color | Blue-green |
| Solubility | Soluble in water |
| Odor | Odorless |
| pH | 3-4 |
| Pictograms : |  |
| Hazard Statements : | H302 Harmful if swallowed H315 Causes skin irritation H319 Causes serious eye irritation |
| Precautionary statements : | P305 + P351 + P338 IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present, and easy to do. Continue rinsing. |
Generally, ferrous sulfate is safe when used at the recommended dose. However, excessive or long-term use can lead to iron overload, which may cause liver damage or hepatotoxicity, especially in individuals with genetic disorders like hemochromatosis.
Lying down immediately may increase the risk of gastrointestinal discomfort or acid reflux. It is recommended to remain upright for at least 30 minutes after ingestion to aid absorption and minimize side effects.
Ferrous sulfate is not known to cause high blood pressure. It is generally considered safe for individuals with normal or elevated blood pressure.
Ferrous sulfate does not directly affect menstruation, but by correcting iron deficiency, it may help reduce heavy menstrual bleeding.
While taking ferrous sulfate, it is advisable to avoid calcium supplements, dairy products, antacids, proton pump inhibitors, and high-fiber foods, as they can interfere with its absorption.
Ferrous sulfate does not cause weight gain directly. However, some people may experience improved appetite as iron levels normalize, which could lead to weight gain if calorie intake also increases.