
Sodium iodide is an ionic compound comprising sodium cations (Na⁺) and iodide anions (I⁻) in a 1:1 ratio. It is a white, water-soluble solid with a crystal lattice structure. Sodium iodide is formed by the reaction between sodium metal and iodine and is produced industrially by reacting acidic iodides with sodium hydroxide. It is classified as a chaotropic salt, which means it can disrupt the structure of water and other solvents.
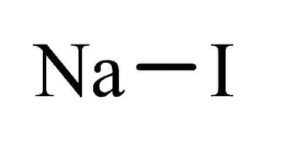
CAS No.: 7681-82-5
Synonyms: Sodium monoiodide; Ioduril; Soiodin; Iodure de sodium; sodiumiodide; Jodid sodny; Natriumjodid; Natrii iodidum.
Properties of Sodium Iodide
| Physical Properties | |
| Chemical formula | NaI |
| IUPAC Name | sodium;iodide |
| Molecular weight | 149.8942 g/mol |
| Solubility | Water, Ethanol & acetone |
| Density | 3.67 g/cm³ |
| Chemical Properties | |
| Color | Colorless to White |
| State | Dry Powder; Liquid; Pellets; Large Crystals |
| Melting point | 651 °C |
| Specific Gravity | 3.67 |
| pH | 8-9.5 |
| pKa | 0.067 at 20 ℃ |
| Pictograms : |  |
| Hazard Statements : | H319: Causes serious eye irritation. |
| Precautionary statements : | P264: Wash skin thoroughly after handling. P280: Wear eye protection/ face protection. P305 + P351 + P338: IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present, and easy to do. Continue rinsing. P337 + P313: If eye irritation persists: Get medical advice/ attention. |
Sodium iodide is an ionic compound comprising sodium cations (Na⁺) and iodide anions (I⁻) in a 1:1 ratio. It is a white, water-soluble solid with a crystal lattice structure. Sodium iodide is formed by the reaction between sodium metal and iodine and is produced industrially by reacting acidic iodides with sodium hydroxide. It is classified as a chaotropic salt, which means it can disrupt the structure of water and other solvents.
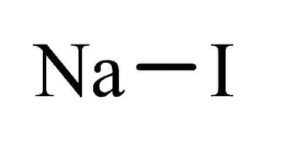
CAS No.: 7681-82-5
Synonyms: Sodium monoiodide; Ioduril; Soiodin; Iodure de sodium; sodiumiodide; Jodid sodny; Natriumjodid; Natrii iodidum.
Properties of Sodium Iodide
| Physical Properties | |
| Chemical formula | NaI |
| IUPAC Name | sodium;iodide |
| Molecular weight | 149.8942 g/mol |
| Solubility | Water, Ethanol & acetone |
| Density | 3.67 g/cm³ |
| Chemical Properties | |
| Color | Colorless to White |
| State | Dry Powder; Liquid; Pellets; Large Crystals |
| Melting point | 651 °C |
| Specific Gravity | 3.67 |
| pH | 8-9.5 |
| pKa | 0.067 at 20 ℃ |
| Pictograms : |  |
| Hazard Statements : | H319: Causes serious eye irritation. |
| Precautionary statements : | P264: Wash skin thoroughly after handling. P280: Wear eye protection/ face protection. P305 + P351 + P338: IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present, and easy to do. Continue rinsing. P337 + P313: If eye irritation persists: Get medical advice/ attention. |
Sodium iodide (NaI) is an ionic compound. It consists of sodium cations (Na⁺) and iodide anions (I⁻), which are held together by strong electrostatic forces of attraction between the oppositely charged ions.
Sodium iodide is commonly used in nutritional supplements, organic synthesis, and nuclear medicine for diagnostic imaging and treatment.
Sodium iodide (NaI) is polar because it is an ionic compound, with a significant difference in electronegativity between sodium (Na) and iodine (I).
To distinguish sodium chloride (NaCl) from sodium iodide (NaI), Add a few drops of silver nitrate (AgNO₃) solution to each sample dissolved in water. Sodium chloride gives a white precipitate whereas sodium iodide gives a yellow one.